Abstract
Background: Pulmonary embolism (PE) is a catastrophic complication in patients with venous thrombosis and is the third leading cardiovascular cause of death after myocardial infraction and stroke. Inflammation, hemostatic dysregulation, and generation of thrombotic mediators contribute to the pathogenesis of PE. Persistent thrombin generation contributes to the overall pathology of PE. The purpose of this study is to investigate endogenous thrombin potential (ETP) in PE patients and demonstrate its relationship with thrombin generation biomarkers, such as prothrombin fragment 1+2 (F1+2), thrombin anti-thrombin complex (TAT) and D-Dimer.
Methods: Citrated blood samples from 201 patients with confirmed PE were collected under an IRB approved protocol. Fifty normal human plasma (NHP) samples were used as a reference. Thrombin generation studies were carried out by using fluorogenic substrate method (calibrated automated thrombogram, Diagnostica Stago; Paris, France). Thrombin generation parameters such as peak thrombin, lag time and endogenous thrombin potential (ETP) were compiled. Plasma samples were analyzed for such biomarkers as prothrombin fragment 1+2 (F1+2), thrombin anti-thrombin complex (TAT), and D-Dimer by using commercially available Sandwich ELISA methods. Results were compiled as mean ± SD and applicable statistical analysis was performed by using GraphPad Prism software.
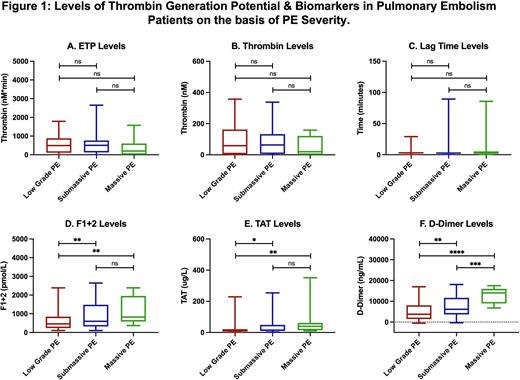
Result: PE patients exhibited a wide variation in thrombin generation parameters. ETP in PE patients decreased to 549.2±508.4 nM*min compared to NHP 701.8±214.6 nM*min, thrombin levels in PE patients also decreased to 83.7±80.1 nM compared to NHP 158.5±31.5 nM, while lag time increased 5.4±12.7 mins to 2.6±0.6 mins compared to NHP. Biomarker analysis showed elevated levels of F1+2 (972.3±1508.0 vs 294.2±105.0 pmol/L, 3.3-fold), TAT (34.9±54.3 vs 2.3±1.1ug/L, 15.3-fold), and D-Dimer (6720.0±4810.0 vs 182.6±205.8 ug/ml, 36.8-fold) compared to NHP. Endogenous thrombin potential levels demonstrate moderate and positive correlations with F1+2 (r = 0.22) while weak correlations were noted with TAT (r = 0.14) and D-Dimer (r = 0.05). F1+2 possess strong correlation with TAT (r = 0.55) and moderate for D-Dimer (r = 0.21). TAT also showed strong correlation with D-Dimer (r = 0.45). Thrombin generation parameters and biomarker levels based on PE severity has been shown in figure 1. Marked increase in thrombin generation biomarker levels were noted with increase PE severity. However, for the thrombin generation parameters, there is no differences were noted between the low grade and sub-massive PE, while the ETP and peak thrombin levels decreased, and lag time increased for the massive PE.
Conclusion: These studies suggest that persistent thrombin generation in PE patients results in the consumption of prothrombin complex. However, such biomarkers of thrombin generation as F1+2, TAT and D-Dimer are formed, leading to higher circulating levels. Simultaneous measurement of thrombin generation biomarkers and thrombin generation parameters may be useful to determine the pathogenesis of thrombotic complications and optimization of treatment options.
Disclosures
Monreal:Leo Pharm: Research Funding; Rovi: Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Other: Presented to congress, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal